Ataxia is a medical condition that impacts the coordination, balance, and speech of an individual. Recent statistics have shown that in the United States alone, around 15,000 to 20,000 people suffer from spinocerebellar ataxia (SCA). The medical condition can develop at any age and is acquired due to underlying conditions such as multiple sclerosis, stroke, tumors, or peripheral neuropathy.
In addition, other factors that contributed to this medical condition include alcoholism and metabolic disorders. Some common symptoms of ataxia include impaired coordination, loss of muscle control, and abnormal postures. Current treatment options for the condition are based on symptom management and are aimed at improving the quality of life. However, research is being conducted to explore stem cell treatment for ataxia. Let’s learn more about this option.
What is ataxia?
Ataxia is a medical condition that is characterized by various coordination disturbances due to damage to the cerebellum, the spinal cord, or peripheral neurons. It can develop at any age depending and may be caused by diseases such as multiple sclerosis and other neurological conditions that damage the central nervous system and result in uncoordinated movements.
The diagnosis is determined based on physical and neurological exams. The process also encompasses CT or MRI of the brain to reveal changes in the cerebellum and blood tests that can reveal vitamin or coenzyme deficiencies. Treatment of ataxia can be challenging due to the potential causes. Current studies have explored new medications and novel approaches, including stem cell treatment for ataxia.
Symptoms and common signs of ataxia
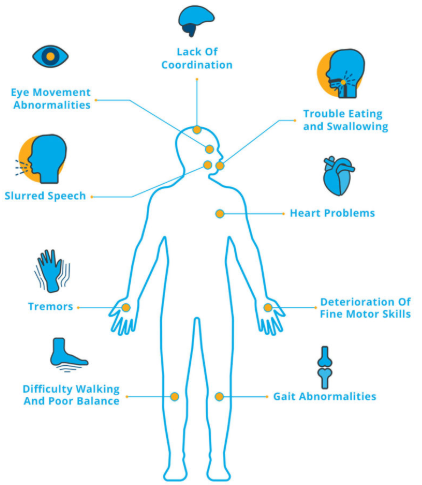
Those suffering from ataxia may experience problems with mobility. Initially, they notice problems with coordination, which may manifest as gait impairment and frequent stumbling. These initial problems with walking may be later accompanied by the following signs and symptoms:
- Hesitation in speaking;
- Problems with handwriting and typing;
- Difficulty with chewing and swallowing;
- Vision and cognitive dysfunctions;
- Fatigue;
- Difficulty with concentration;
- Heart problems;
- Acute headache, nausea, and vomiting.
In most cases, symptoms of ataxia get worse with time, significantly deteriorating the quality of life and normal functioning of the patient.
Types of ataxia
The condition may be inherited, acquired, or idiopathic. There are a number of different factors that can lead to acquired or idiopathic ataxia, which may include damage to the brain or excessive composition of alcohol.
Inherited ataxia
This type of ataxia includes dozens of rare heterogeneous neurological diseases, such as spinocerebellar ataxias, Friedreich ataxia, and ataxia telangiectasia. They can occur due to a failure of one or more genes and passed through generations.
Acquired ataxia
This is caused by damage to the brain or spinal cord due to disease, injury, or exposure to alcohol or certain drugs. Symptoms for acquired ataxia tend to develop due to a stroke or nutritional deficiencies.
Idiopathic ataxia
This includes all ataxia with unknown causes and is also known as cerebellar ataxia.
Modern approaches to ataxia treatment
When the treatment of the underlying disease is available, the symptoms of ataxia may be completely or partly cured, or the progression of the disease may halt. For example, in the case of resectable tumors and vitamin and coenzyme deficiencies, patients recover in 6 months to a year after the underlying cause is treated.
Several ongoing studies showed partial improvement of ataxia symptoms in patients with SCA treated with lithium or varenicline 12. Improvement was also seen when using riluzole in patients with cerebellar ataxia of different etiologies. In addition, advancements are being made in the use of stem cell treatment for cerebellar ataxia.
Symptomatic treatment is used to relieve muscle spasms, tremors, bladder problems, abnormal eye movements and depression, as well as for cardiac problems seen in Friedreich’s ataxia. Other dysfunctions such as fatigue and neuropathy are also managed with the relevant medications.
Physiotherapy and physical exercises play a significant role in increasing quality of life as they may help to preserve mobility and increase muscle strength. Speech and language therapists can help to improve speaking and communication problems, difficulties with swallowing, coughing and choking. An occupational therapist can also be helpful, for example, with home adaptations, teaching strategies for daily activities or wheelchair assessments.
Stem cell treatment for ataxia is becoming a promising option for medical scientists that are still searching for the most effective therapy to prevent the onset or inhibit the progression of this condition.
Contact us
Get a free online consultation from our medical advisors to find out more about the benefits of stem cells for your personal case.

Medical Advisor, Swiss Medica doctor
Scientific evidence of stem cell therapeutic effect on ataxia
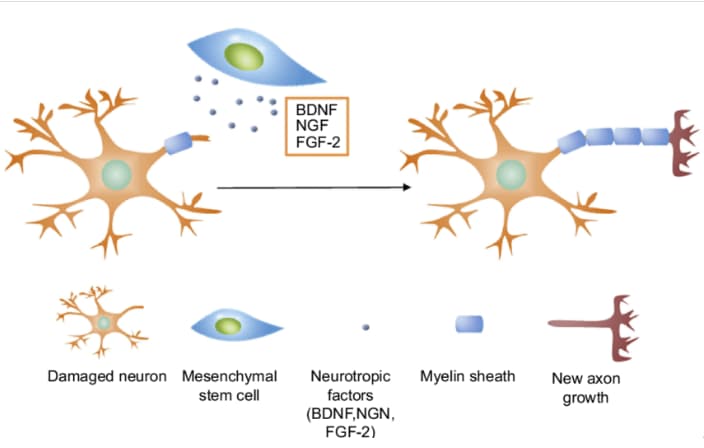
Recent studies demonstrate that stem cell ataxia treatment is easily tolerated and can provide remarkable improvements in patients. It was shown that the use of mesenchymal stem cells (MSCs) during stem cell therapy for cerebellar ataxia is partially effective.
It is assumed that degenerating neurons attract injected multipotent mesenchymal stem cells (MSCs) and connect with them. The MSCs then directly provide neuronal growth factors to cells that are being destroyed to rescue them from degeneration. It was also shown that these types of cells induce synaptic connections and reduce apoptosis in neurons. For example, a single injection of MSCs in mice with spinocerebellar ataxia positively affected the progressive degeneration of both axons and myelin in the spinal cord. Given this, it can be stated that stem cell ataxia treatment may alleviate symptoms and improve quality of life.
Expected improvements
Treatment results in those suffering from ataxia occur due to the neuroprotective, anti-inflammatory, and neurotrophic effects of MSCs. These include the following:
- Improvements in coordination and balance.
- Increased strength in the limbs and muscles.
- Improvement of fine motor skills and speech.
- Reduction of fatigue.
- Overall increased quality of life.
Results of Swiss Medica patients
Swiss Medica offers complex neurological rehabilitation for patients with ataxia due to different health issues. The causes of the condition are diverse, and therefore, the approach used for stem cell therapy for adult ataxia and treatment options for underaged patients are customized based on each individual case.
Feedback from a post-stroke patient treated with stem cells
“I had absolutely no expectations. But after two days of treatment, small miracles began to happen. The spasticity in my left leg disappeared, the strength of my left hand equated to my right hand, no more weakness in my left hip, my walking improved by 70%. What I am most looking forward to is to return to Australia and going back to my doctors and neurologists who told me that stem cell treatment for strokes was a waste of time. I’d love to look at their faces when I walk in”.
Patient from Australia.
Diagnosis: Stroke in the medulla.
Duration: 4 years.
Before the treatment:
- Weakness at the left-hand side of the body;
- Unable to walk.
After the treatment:
- No spasticity and weakness in the limbs;
- Walking improved by 70%.
Feedback from a patient with spinocerebellar ataxia, type 2, inherited
“Since we came here in June, he has improved tremendously, and he’s changed his whole lifestyle down to diet, exercise, everything. And his complete well-begin, I would say, in my opinion, probably 20% to 30% improved from before.”
Patient from Ireland.
Diagnosis: spinocerebellar ataxia, type 2, inherited.
Duration: 7 years.
Before the treatment:
- Problem with balance;
- Walking problems;
- Insomnia occasionally;
- Slight problem with swallowing.
After the treatment:
- 20% to 30% improved quality of life;
- Changes in diet and exercise.
More video testimonials are available on our YouTube channel.
Ataxia treatment with stem cells: the procedure
Stem cell treatment options for ataxia at Swiss Medica include intrathecal or intravenous administration of the patient’s own autologous bone marrow cells combined with placental and umbilical cord cells. The procedure of collecting cells from a patient is preceded by local anesthesia.
| A | B |
Cells taken from the patient’s bone marrow undergo several steps before being administered. They are processed, separated using a centrifuge, cultivated if required, and bioactivated to get specific cell products for optimal effects. Donor cell-based products are already prepared and can be introduced immediately.
Cells are then delivered into the body intrathecally. The treatment process in the clinic lasts for around 10 days, and the effect may be observed within 15-40 days. It is recommended to repeat the course after 6 months to maintain the results and get better improvements.
Indications and contraindications for stem cell therapy
This approach may be beneficial to those patients who suffer from diseases or medical conditions accompanied by symptoms of ataxia. Such medical conditions include:
- Multiple sclerosis
- Parkinson’s disease
- Head injury
- Sarcoidosis
- Celiac disease
- Toxic reaction, etc.
However, there are some limitations that can hinder the positive effects of stem cell treatment or are considered direct contraindications, such as any life-threatening or terminal health conditions (including cancer or tumors) and past negative experiences with cell products. Other contraindications for stem cell therapy are:
- Infectious disease in the acute stage.
- Stroke or transient ischemic attack in the last 3 months.
- Deviations of some indicators in blood tests.
- Pregnancy and lactation.
- Mental disorders and addictions.
- Contraindications to anesthesia and/or high risk of bleeding and/or pathological processes in the area of the proposed biopsy (does not exclude the possibility of using donor cell products) and some others.
Safety of stem cell therapy and possible side effects
The safety of stem cell treatment has been shown in several studies related to patients with different pathologies. Studies have shown that stem cell treatment for cerebellar ataxia has not led to any adverse events.
Most patients tolerate the procedure well. Short-term and transient fever during the procedure, while rare, cannot be excluded. Swiss Medica specialists will monitor your condition for safer and more beneficial results.
What does the treatment include?
Due to a complex factor standing behind each case of ataxia, additional therapies may be required. The patient’s neurological status, main diagnoses, age, family history, and other aspects are considered when the individual therapy plan is developed.
Swiss Medica offers potent options to improve the results of stem cell treatment in patients suffering from ataxia. The following procedures are advised for such patients:
- Kinesiotherapy;
- Electrical myostimulation;
- Interval hypoxic hyperoxic treatment;
- Mesodiencephalic modulation (transcranial electrostimulation of the brain).
Consultations with specialists in interfacing areas such as neurorehabilitation, psychology, and nutrition are also available.
Cost of stem cell treatment options for ataxia
The cost of stem cell therapy for ataxia is based on several factors, such as diagnosis and feasible treatment options, and may vary from one patient to the other.
Contact us
Book a free online consultation today and learn more about the results, duration, and cost of stem cell treatment for ataxia.

Medical Advisor, Swiss Medica doctor
List of References:
Saute JA et al. A randomized, phase 2 clinical trial of lithium carbonate in Machado-Joseph disease. Mov Disord. 2014 Apr; 29(4):568-73.
Zesiewicz TA et al. A randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3. Neurology. 2012 Feb 21; 78(8):545-50.
Ristori G et al. Riluzole in cerebellar ataxia: a randomized, double-blind, placebo-controlled pilot trial. Neurology. 2010 Mar 9; 74(10):839-45.
Tsai Y-A et al. Treatment of Spinocerebellar Ataxia with Mesenchymal Stem Cells: A Phase I/IIa Clinical Study. Cell Transplant. 2017 Mar; 26(3): 503–512.
Nakamura K, Mieda T, Suto N, Matsuura S, Hirai H. Mesenchymal stem cells as a potential therapeutic tool for spinocerebellar ataxia. Cerebellum. 2015 Apr;14(2):165-70.
Mieda T et al. Mesenchymal stem cells attenuate peripheral neuronal degeneration in spinocerebellar ataxia type 1 knockin mice. J Neurosci Res. 2016 Mar;94(3):246-52.
Goldman SA. Stem and progenitor cell-based therapy of the central nervous system: Hopes, hype and wishful thinking. Cell Stem Cell. 2016 Feb 4; 18(2): 174–188.
Kwon S, Yoo KH, Sym SJ, Khang D. Mesenchymal stem cell therapy assisted by nanotechnology: a possible combinational treatment for brain tumor and central nerve regeneration. Int J Nanomedicine. 2019 Jul 29;14:5925-5942.
Venkataramana NK et al. Bilateral transplantation of allogenic adult human bone marrow-derived mesenchymal stem cells into the subventricular zone of Parkinson’s disease: a pilot clinical study. Stem Cells Int. 2012;2012:931902.
Huang NF and Li S. Mesenchymal stem cells for vascular regeneration. Regen Med. 2008 Nov; 3(6): 877–892.
Ciervo Y, Ning K, Jun X, Shaw PJ, Mead RJ. Advances, challenges and future directions for stem cell therapy in amyotrophic lateral sclerosis. Mol Neurodegener. 2017 Nov 13;12(1):85.
Dahbour et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci Ther. 2017 Nov; 23(11): 866–874.
Hare JM. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86.
Medical Advisor, Swiss Medica doctor








