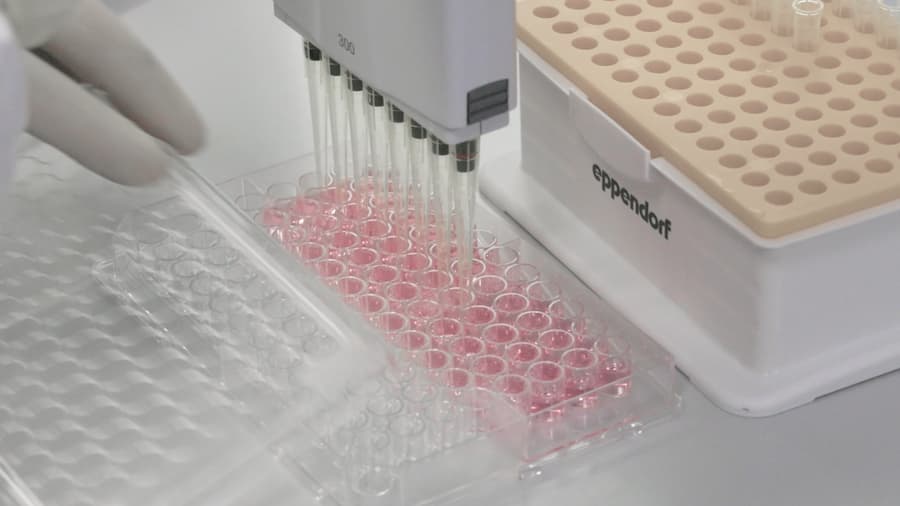
Welcome to Swiss Medica clinic, where innovative stem cell therapy meets advanced patient care. Our laboratory is dedicated to producing and storing high-quality stem cells to ensure improvements for our patients. Here’s a guide to our stem cell production process and what makes us a leader in the field.
Major Principles of Our Production
We strive to provide our patients with the highest quality cell-based products possible, and we monitor every stage of production in our laboratory. As a result, three guiding principles govern everything we do.
Cell Efficiency
Our stem cell production process is well-designed to maximize cell efficiency. This means that the stem cells we produce are highly potent and capable of regenerating damaged tissues effectively.
Here is what we do to make it possible:
- The laboratory team controls each dose by checking the vitality and the number of cells.
- We provide high yields and efficiency of stem cells through modern equipment such as dewars for long-term storage, spacious incubators for safe growth and active multiplication of cells, centrifuges for high-quality cell isolation, and others.
- In most cases, we use fresh activated cells. This means that if the cells are frozen, we not only defrost and use them right away, but rather, we activate them to maximize their efficiency.
- We use different types of cells to treat various types of conditions. For example, our laboratory professionals turn mononuclear cells into mesenchymal stem cells (MSCs), or neural stem cells, so there are more treatment options.
For detailed information about the different cell and cell-free therapies we offer, visit this page.
Go to the Therapies section
Production Safety
We monitor production safety at each stage.
Screening and assessment
Whether MSCs come from a donor or the patient, before being utilized in treatments, they are subjected to extensive testing to ensure they are pure, viable, and sterile.
These tests include:
- Screening for bacterial, fungal, and viral contamination,
- Genetic and molecular analyses to verify their identity and functionality.
Extraction procedures
We harvest high-quality stem cells with minimal discomfort for the donor or patient.
Cell isolation and purification
This ensures that only the most potent and viable stem cells are used in treatment, maximizing therapeutic outcomes.
Processing and culturing
We carefully monitor every stage of production to ensure that the quantity and quality of stem cells meet the exact dosage specified in the patient’s treatment plan.
Laboratory Sterility
With an in-house laboratory, we can process and prepare stem cells for treatment quickly, so there are no issues with transportation, timing, or possible contamination. Our laboratory obtained a Grade A cleanliness level, which means the cleanest environment and surfaces that can be used for sterile operations.
We divided it into two sections—for preparatory procedures and operations with checked cells—to reduce the risk of cross-contamination.

In-house laboratory professionals test the laboratory environment for sterility once a month, and an independent laboratory does the same at least quarterly.
Curious to learn more about the advanced capabilities of our in-house laboratory? Dive into our detailed article here.
Read nowInternational Standards
Our licensed doctors and healthcare professionals have 12+ years of experience in regenerative medicine. Swiss Medica’s medical facilities in Serbia adhere to the Serbian legal and regulatory framework, applicable European and Serbian healthcare standards, and strict ethical guidelines to ensure patients’ safety and treatment effectiveness.
For more details about our facilities, the conditions we treat, our stem cell products, and what to expect during your visit, please visit this page.
More about Swiss MedicaContact us
Swiss Medica focuses on safety and efficacy at every step. Contact us today for a free, no-obligation consultation to know why you can trust our stem cell products.

Medical Advisor, Swiss Medica doctor
The Production of Stem Cells from A to Z
Our experts use high-quality equipment and technologies to monitor each stage of the production process.
How we get stem cells
We ensure that cells come from donors who volunteered to donate them following a healthy childbirth. We have access to test results and the pregnant woman’s medical history to ensure that cells are obtained from healthy adults who do not have chronic diseases or pregnancy complications. Our donor stem cells might come from:
- Placental tissue (tissue that supports fetal development);
- Umbilical cord tissue.
We also sample and derive stem cells from patients in our in-house laboratory when our doctors recommend it. Before using a stem cell product in a patient’s treatment, we thoroughly inspect it several times to ensure that it meets safety requirements.
With our in-house laboratory, we can closely monitor stem cells’ composition and viability, guaranteeing safe and effective doses. For example, to ensure their safety, all cells are processed in a laminar flow biosafety cabinet and are transported to the surgery room via the UV disinfection airlock.
Patient evaluation and assessment
We encourage our patients to book a no-obligation consultation with our doctors and discuss the potential benefits of stem cell therapies for their conditions. Our medical team carefully evaluates the medical history of the patient and suggests possible treatment options and potential sources of stem cells.
This article takes you through the entire process of stem cell therapy from start to finish.
Read nowCell Extraction
Here are the types of stem cells we extract from the patient’s tissues:
- Adult multipotent mesenchymal stem cells (MSCs) from adipose tissue (SVF), also known as body fat, bone marrow, gingiva, and some other sources.
- Mature specialized cells, for example, macrophages, regulatory T lymphocytes (Tregs), fibroblasts, and some others.
We extract MSCs due to their high safety profile, immunomodulatory, and anti-inflammatory properties. MSCs are harvested using minimally invasive procedures under local or general anesthesia.
Explore a wide range of stem cell therapies that we offer at Swiss Medica.
Visit the sectionCell Isolation, Purification and Expansion
The harvested cells are isolated and purified in a laboratory to obtain a high concentration of viable stem cells.
After the first stage of purification, stem cells are stored in culture flasks and plates in cell incubators. These incubators maintain a warm temperature (approx. 37 °C), appropriate humidity, and a low CO2 level (approx. 5%) to mimic the conditions within the body. Stem cells receive growth factors and nutrients, which promote their growth and multiplication.
When the cells reach a certain stage of activity, they are collected using chemical reagents. Then, with the application of a centrifuge, they are isolated and purified.
We also produce secretome and exosomes in our lab. They are extracted from the environment in which the cells are cultured. Once only the cells remain, they are counted to ensure the correct dosage and their viability.
Secretome and exosome therapies are typically less invasive, as they deliver molecular signals rather than live cells. To learn more about these innovative treatments, read this article.
Read more
Cryopreservation (not always required)
Cryopreservation is required when stem cells are to be stored for later use. This may be necessary for several reasons:
- Stem cells can be collected from a patient before they are needed for therapy. Cryopreserving the cells allows them to be kept until they are needed for treatment.
- In cases where multiple treatments or doses of stem cells are required, cryopreservation allows excess cells to be stored for later use. It eliminates the need for repeated harvesting procedures.
- Cryopreservation enables the storage of stem cells from a donor for use in allogeneic transplantation, where the cells are transferred to a different individual.

This is how freezing stem cells for later use is done.
- When the cells reach a certain level of activity, they are collected with chemical reagents. They are then separated and purified using a centrifuge.
- We mix isolated cells with a liquid called cryoprotectant that helps protect them during freezing.
- After that, we place them in tiny containers called cryovials and put the freezer under the precise control of computer software. To keep the cells alive, we cool the tubes following a specific plan. We make sure they cool down slowly, about 1–2 degrees per minute.
- At the end of the freezing program, the cryoprobes are placed in liquid nitrogen. Under these conditions, cells can be stored for an unlimited period of time.
Stromal cells maintain their therapeutic potential even after cryopreservation.
Cell Proliferation Monitoring
The laboratory team constantly monitors:
- Cell viability to know there are live and functional cells, using techniques such as trypan blue exclusion or flow cytometry.
- Proliferation rate to know how quickly the cells are dividing. This rate can be increased or slowed down by adjusting the growth conditions.
- Accurate number of cells. We carefully measure the exact number of stem cells required for each individual case, leaving nothing to chance.
We closely monitor each step of the production process.
Quality and Safety Control
Stem cells are subjected to a variety of tests to ensure their safety and efficiency in order to avoid any negative effects or serious conditions. We check them at several stages, including before administration.
- Quality control ensures the therapeutic potential of cells and includes characteristics like surface markers, genetic stability, and differentiation potential.
- Safety control includes tests for sterility and possible infections, such as HIV, hepatitis B, C, herpes, CMV, toxoplasma, mycoplasma, endotoxins, and others.
Storage and Injection
After all tests, a portion of the patient’s cells may be diluted with saline and then injected into the patient’s body under sterile conditions in the clinic.
Another part could be cryopreserved in liquid nitrogen in cryogenic storage dewars until the patient’s next visit. After thawing, we simply activate these cells. For that, we don’t move them right after thawing. Instead, we wait a while for them to “wake up” and start growing again. This gives us time to check if they handled the freezing okay and are ready to be used.
Stem cells are prepared for storage.
The preparation time for the donor stem cell is usually shorter as there are no collection, freezing, or storing procedures before the treatment.
Contact us
We focus on every detail, from sourcing to final preparation, to ensure effective treatments. Fill out the form to schedule a no-obligation consultation and find out how our stem cell products can benefit you.

Medical Advisor, Swiss Medica doctor
Ready to Explore More?
Discover the full potential of stem cells and dive into additional insightful articles.
List of References
Wang, Y., Yi, H. & Song, Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther 12, 545 (2021). https://doi.org/10.1186/s13287-021-02609-x