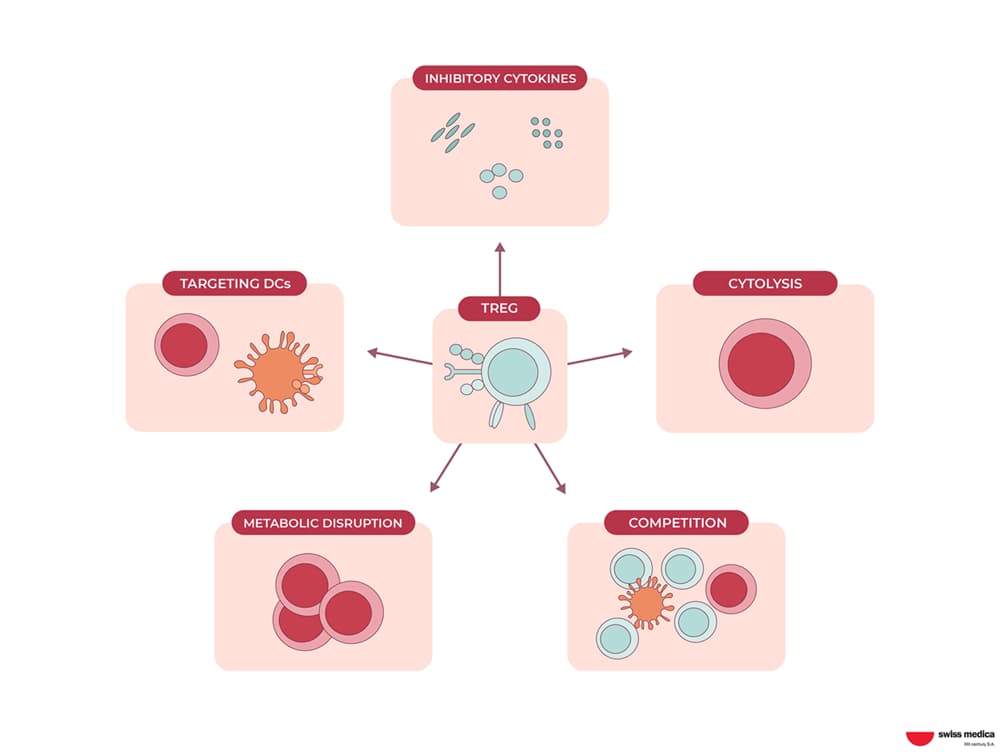
Regulatory T-cells, or T-Regs, are a type of T-cells that plays an important role in immune balance. They serve as the body’s peacekeepers, preventing the immune system from reacting excessively and attacking healthy tissues. Consider them traffic cops directing the immune system’s response. Thus, their functions are:
Preventing overreaction
T-Regs inhibit the activity of other immune cells, such as T-cells and B-cells, preventing them from attacking healthy tissues. This is critical in preventing autoimmune diseases, in which the immune system mistakenly attacks the body’s own cells.
Maintaining tolerance
T-Regs assist the immune system in distinguishing between foreign invaders and the body’s own cells. This has the potential to reduce transplant rejection.
Suppressing inflammation
T-Regs produce molecules that reduce inflammation, a natural immune response that can occasionally become excessive and harmful.
When Do We Use T-Reg Cells
Some autoimmune diseases exhibit a noticeable decrease in T-Reg functions, which contributes to the dysregulated immune response and inflammation associated with these conditions:
- Multiple Sclerosis (MS)
- Inflammatory Bowel Disease (IBD)
- Type 1 diabetes
- Crohn’s disease
- Injures
Regulatory T-cells may be useful in treating these diseases because they aim to restore or enhance T-regulatory functions. This could potentially reduce the immune-mediated pathology associated with these diseases.
We only use autologous cells, or cells from the patient’s own body, for regulatory T-cell therapy
Get a free online consultation
If you have one of the conditions listed above and are exploring alternative therapy options, contact us to schedule a no-obligation online consultation. Our medical advisors will help determine if T-reg therapy is suitable for you or recommend other treatments available at Swiss Medica.

Medical Advisor, Swiss Medica doctor
Differences from Mesenchymal Stromal Cells (MSCs)
MSCs and T-Regs are two distinct cell types that serve different functions. We administer T-Regs to patients who have a T-Reg deficiency. In these conditions, T-Reg cells are crucial for controlling the immune system and preventing it from attacking the body’s own tissues.
MSCs, on the other hand, are usable regardless of their concentration in the patient’s body. The combination of T-regs and MCSs leads to more consistent results after stem cell treatment.
Missed the details on MSCs?
We’ve got you covered. Check out this article for all the information.
Before using T-reg therapy, we perform an immunogram to detect and record any decrease in the patient’s regulatory T-cell levels. This aids in the assessment of the patient’s immune status and the need for this treatment.
T-Reg Cells Collection and Preparation
This process requires the patient to visit the clinic twice: once for blood collection and later for the infusion of the prepared T-reg cells.
- We collect approximately 40-60 mL of peripheral blood from the patient.
- We keep these cells frozen until the next patient’s arrival. Cryopreservation preserves their viability and functionality, allowing for long-term storage and future use.
- Prior to the patient’s arrival, we assess the state of the cells, activate and culture them until the required number is reached. It takes approximately 15 to 21 days.

Want to learn more about our services and travel arrangements to the clinic? Discover all the details in our article.
Find out moreHow Do We Deliver T-Reg Cells
Before the therapy, we run various tests to assess the patient’s overall health and determine whether there are any contraindications to the procedure.
We use two ways to deliver the cells:
- Intravenous administration involves injecting the cells directly into a vein, which allows them to circulate throughout the body via the bloodstream.
- Subcutaneous administration involves injecting the cells into the tissue layer just beneath the skin, where they can gradually absorb into the bloodstream.
We have a dedicated page that explains in detail how stem cells can be administered to your body.
Take a lookDosages
Initially, the treatment begins with a standard dose of 100 million cells, which is the minimum dose required to see the results of the treatment and progression thereafter.
Subsequent dosages, or titrations, can be adjusted based on the patient’s weight, ranging from 0.5 to 2 million cells per kilogram of body weight. This titration enables personalized dosing based on each individual’s needs.
Contraindications for T-Reg Cells
There are specific conditions that make this treatment unsuitable:
- Oncological diseases
- Chronic viral infections, including those in incubation period, such as HIV and hepatitis
- Recent contact with infectious individuals within the past 4 weeks
- Pregnancy
- Continuous usage of glucocorticosteroids
- Normal levels of T-Reg cells, which indicate a properly functioning immune system
Expected T-Reg Cell Treatment Results
The treatment’s therapeutic effect begins within 4 weeks of administration and can last up to a year. These time periods vary from patient to patient.
Contact us
To discuss your condition, explore treatment options, and understand potential outcomes, contact us to schedule a free online consultation with our medical advisors.

Medical Advisor, Swiss Medica doctor
Ready to Explore More?
Discover the full potential of stem cells and dive into additional articles.
List of References
Bluestone JA, McKenzie BS, Beilke J, Ramsdell F. Opportunities for Treg cell therapy for the treatment of human disease. Front Immunol. 2023 Apr 19;14:1166135. doi.org/10.3389/fimmu.2023.1166135
Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015 Aug 27;162(5):1078-89. doi.org/10.1016/j.cell.2015.08.021
Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004 Jun 7;199(11):1455-65. doi.org/10.1084/jem.20040139