Neural stem cells (NSCs) are self-renewing, multipotent cells found in the central nervous system (CNS). Due to their neuroprotection and immunomodulation properties, NSCs may promote neural repair and regeneration.
At Swiss Medica, we only use the patient’s own NSCs, so there is no need for immunosuppressive drugs.
NSCs Advantages for Neurological Conditions
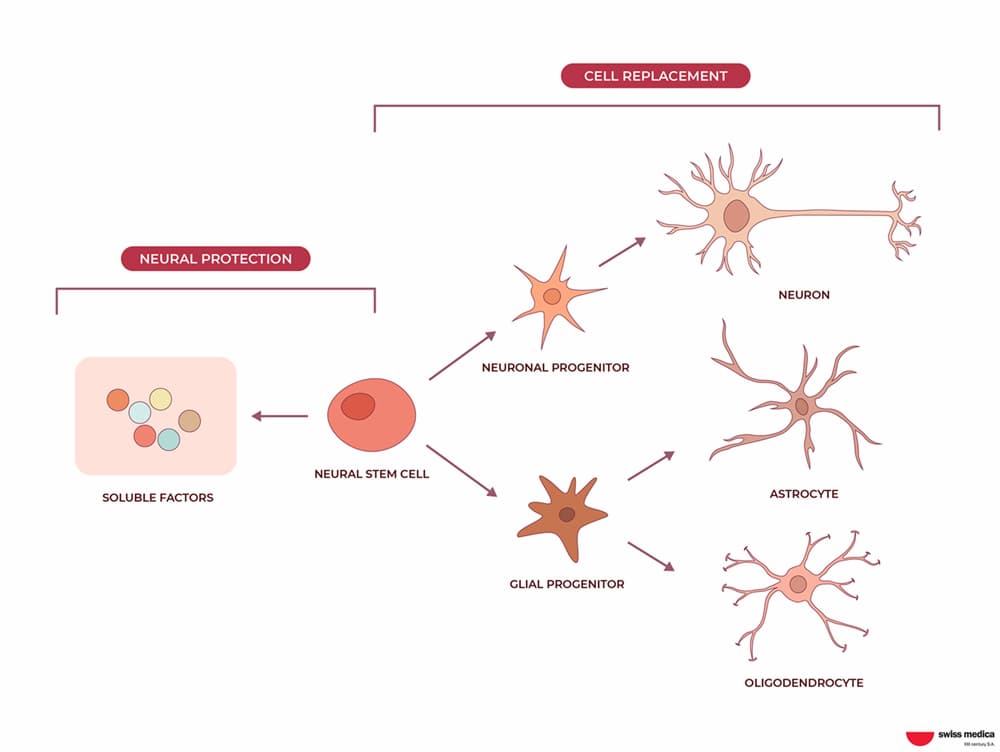
NSCs have the great ability to multiply and create progeny cells. The latter in turn differentiates into neurons, astrocytes, and oligodendrocytes, which are important for the correct functioning and repair of the CNS.
| Neurons or nerve cells | Astrocytes | Oligodendrocytes |
| They are responsible for carrying information from the external world through the sense organs (such as the eyes and ears) to the brain, using electrical and chemical signals. | They are responsible for metabolic, homeostatic, and neuroprotective tasks processes such as regulation of blood flow and control of sleep. Astrocytes act as bridges among neurons, oligodendrocytes, and other cells in the CNS. | These are cells of the CNS that contain a lot of fat that creates a cover around nerves and play an important role in neuronal communication. |
When Do We Use Neural Stem Cells
We apply neural stem cells therapy for a variety of conditions:
| Multiple Sclerosis (MS) | NSCs can help repair damaged myelin and support neuronal function. |
| Trauma | NSCs can promote the regeneration of damaged nerve tissue after injuries. |
| Stroke | NSCs can contribute to the repair and recovery of brain tissue affected by stroke. |
There is also a potential to use NSCs therapy for:
| Parkinson’s disease | Research shows that NSCs may potentially replace lost dopaminergic neurons. |
| ALS (Amyotrophic Lateral Sclerosis) | NSCs may slow down the progression of the neurodegenerative disease. |
| Alzheimer’s disease | NSCs may potentially replace damaged neurons and enhance cognitive function. |
Get a free online consultation
Could your condition be treated with neural stem cells? Contact us to schedule a no-obligation online consultation. Our medical advisors will help determine if this type of therapy is suitable for you or offer an alternative treatment.

Medical Advisor, Swiss Medica doctor
Advantages of Using NSCs Over MSCs in Nerve Tissue Regeneration
Better repair capabilities
NSCs are more effective in repairing damaged nerve tissue compared to MSCs.
Superior regeneration
NSCs have better regenerative effects than MSCs due to their specialized nature.
Broader range of repair capabilities
NSCs can differentiate into both neurons and glial cells, while MSCs may have limited differentiation abilities. This way, NSCs may replace or repair more types of cells in the nervous system.
High Differentiation Rate
In laboratory settings, up to 90% of NSCs differentiate into neurons, while only 10% of MSCs typically may do this in the body.
Learn more about the benefits and applications of MSCs in our detailed article.
Read moreNSCs Collection and Preparation
At Swiss Medica, we only use autologous (the patient’s own) NSCs, which reduces the risk of immune rejection and related complications. Due to the lack of extensive research on the safety and efficiency of NSCs therapy in children, we do not employ these therapies for pediatric cases.
Discover the differences between autologous and allogeneic cells in our article.
Compare nowThis process involves two visits to the clinic: one to perform a bone marrow aspiration and another for the injection of the prepared NSCs.
- We obtain the cells needed for treatment by isolating MSCs from the patient’s bone marrow.
- These cells can be frozen for long-term storage and future use if needed.
- Once the patient’s arrival is scheduled, cells are then cultured in a laboratory until there are enough for the procedure. The process of cultivation and modulation lasts approximately 1.5-2 months until they are suitable for therapeutic use. To ensure an adequate supply, we recommend maintaining a two-month reserve.

How Do We Deliver the NSCs
At Swiss Medica, we administer neural stem cell therapy intrathecally—into the spinal canal.
Intrathecal injection is a safe, efficient, and well-studied method that carries fewer risks of invasiveness and complications. Also, cells can easily travel through the cerebrospinal fluid and potentially reach multiple areas of the CNS, making it ideal for managing central nervous system conditions.
We do not apply NSCs directly at the site of the lesion, or the damaged area. Administering cells this way has more technical challenges and risks, such as introducing infections, damage to surrounding tissues, as well as extended post-operative care and hospitalization.
Explore the various ways stem cells can be delivered with our detailed guide, complete with pictures for a clearer understanding.
Discover nowDosages
For intrathecal administration, the typical dosage ranges from 50 to 70 million cells per procedure, with an average of 60 million cells diluted in 3 ml of solution.
Program Duration
The NSCs treatment program lasts for 4–5 days and can be repeated after one year if necessary.
The program includes the following procedures:
Day 1: Evaluation of the patient’s condition and suitability for the treatment by our healthcare professionals.
Day 2: Administration of the NSCs cells intrathecally. The doctor performs the procedure using intrathecal kit and pencil point spinal needle and a local anesthetic to numb the skin.
Days 3 and 4: Monitoring for any adverse reactions or signs of improvement.
Curious about the complete process and all the steps involved in stem cell therapy? Discover the full details in our article.
Read the full articleCombining with other cells
The NSCs therapy can also be combined with other types of cells, such as cells from the placenta, umbilical cord, and exosomes. This way, we may improve the therapeutic effects and support the regeneration process.
Read more about the exosome therapy we offer at Swiss Medica in this article.
Read nowContraindications
Generally, the treatment with NSCs is well-tolerated and available for many patients. However, there are some contraindications to the procedure:
| Patients under the age of 18 | Due to insufficient research and safety data in children. |
| Patients taking anticoagulants | Anticoagulants can interfere with the body’s ability to clot blood, posing risks during or after the treatment. However, the procedure may be an option if it is possible to discontinue the drug. |
| Patients with some blood clotting disorders | In this case, the procedure should be taken with caution, as the risk of complications increases. |
Treatment Results
After six months: positive effects of the neural stem cells therapy are expected to increase.
After one year: the effectiveness of the treatment may reduce.
It is important to note that treatment results vary depending on the severity of the condition, the duration of the disease, and individual characteristics. For traumatic injuries, a single treatment may be sufficient.
However, neurodegenerative diseases, such as MS or Parkinson’s disease, may require repeated treatments to support or improve the patient’s condition.
Side Effects
Although cell-based therapies cause no long-term side effects, some mild short-term effects are possible and include:
- Fever,
- Headache,
- Chills,
- Rash,
- Localized swelling or redness at the site of the injection.
These effects may be solved with painkillers or taking a rest after the procedure, and disappear within 24 hours. The likelihood of benign tumors developing as a side effect is below 1%. Finally, the cells do not migrate to other body systems, minimizing potential impacts on the patient’s health.
In this article, we’ve debunked the 5 most common myths about stem cell therapy and its side effects. Dive in to see if you’ve heard any of these before.
Find out nowContact us
If you still have any concerns or personal questions about the potential side effects of stem cell therapy, we invite you to discuss them with a medical advisor at Swiss Medica. Don’t hesitate to reach out for a personalized consultation.

Medical Advisor, Swiss Medica doctor
Ready to Explore More?
Discover the full potential of stem cells and dive into additional articles.
List of References
Guo Wen , Zhang Xindan , Zhai Jiliang , Xue Jiajia. The roles and applications of neural stem cells in spinal cord injury repair. Frontiers in Bioengineering and Biotechnology, VOL.10, 2022, https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2022.966866, DOI: 10.3389/fbioe.2022.966866
Feng C, Deng L, Yong YY, Wu JM, Qin DL, Yu L, Zhou XG, Wu AG. The Application of Biomaterials in Spinal Cord Injury. Int J Mol Sci. 2023 Jan 3;24(1):816. doi: 10.3390/ijms24010816. PMID: 36614259; PMCID: PMC9821025.
Baker EW, Kinder HA, West FD. Neural stem cell therapy for stroke: A multimechanistic approach to restoring neurological function. Brain Behav. 2019 Mar;9(3):e01214. doi: 10.1002/brb3.1214. Epub 2019 Feb 12. PMID: 30747485; PMCID: PMC6422715.
Oz T, Kaushik A, Kujawska M. Neural stem cells for Parkinson’s disease management: Challenges, nanobased support, and prospects. World J Stem Cells. 2023 Jul 26;15(7):687-700. doi: 10.4252/wjsc.v15.i7.687. PMID: 37545757; PMCID: PMC10401423.
Vasques JF, Teixeira Pinheiro LC, de Jesus Gonçalves RG, et al. Cell-based Research and Therapy for Amyotrophic Lateral Sclerosis: Promises and Challenges. In: Araki T, editor. Amyotrophic Lateral Sclerosis [Internet]. Brisbane (AU): Exon Publications; 2021 Jul 25. Chapter 7. Available from: https://www.ncbi.nlm.nih.gov/books/NBK573430/ doi: 10.36255/exonpublications.amyotrophiclateralsclerosis.celltherapy.2021
Chen X, Jiang S, Wang R, Bao X, Li Y. Neural Stem Cells in the Treatment of Alzheimer’s Disease: Current Status, Challenges, and Future Prospects. J Alzheimers Dis. 2023;94(s1):S173-S186. doi: 10.3233/JAD-220721. PMID: 36336934; PMCID: PMC10473082.