Some life-threatening conditions, such as blood disorders and certain kinds of autoimmune diseases, are still a challenge for healthcare providers. For some patients who have shown resistance to other treatments, stem cell treatment procedure can offer a potential solution.
So, what is the process of stem cell therapy? And who is best suited for such treatment? This article provides all the answers that you need to start your journey.
What Is a Stem Cell Transplant?
Stem cell treatment procedure includes the creation of a cell product and its subsequent transplantation. During the initial consultation, the doctor assesses the patient’s condition and decides on the type of cell product to use, the source of the cells, and the route of its administration.
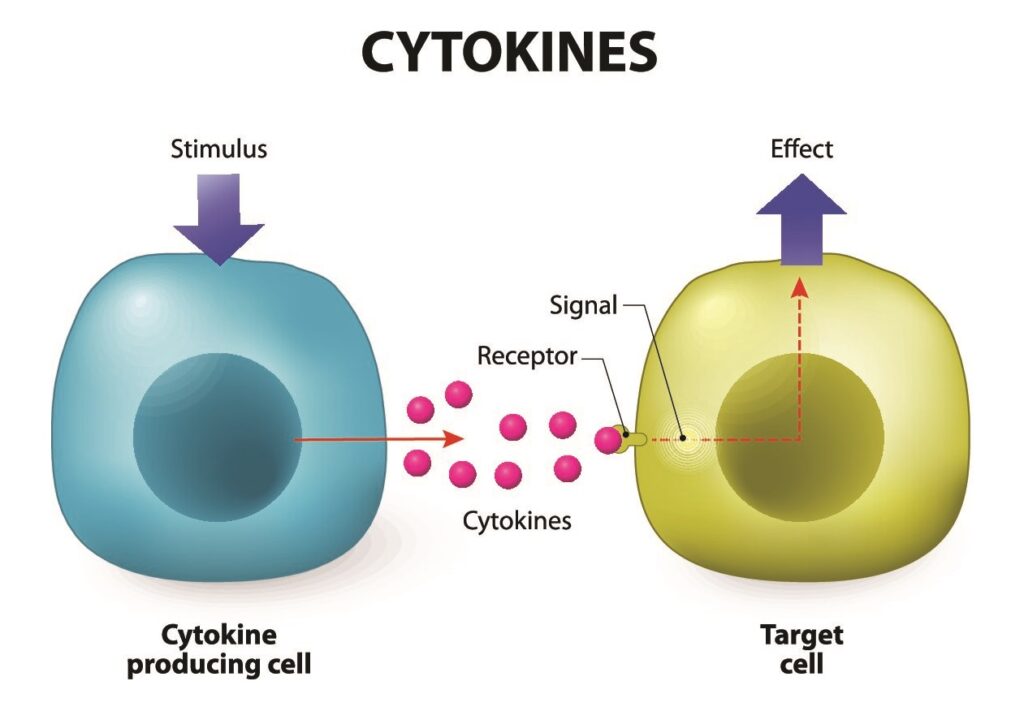
Stem cells are known for their potential to turn into many different cell types in the body. Moreover, stem cells also affect the surrounding tissues, stimulating their recovery (the paracrine effect). Multipotent mesenchymal stromal cells (MMSCs) are those that are most commonly used in stem cell transplants.
At Swiss Medica, patient safety is our highest priority. We only use adult multipotent MSC to avoid possible adverse effects associated with embryonic stem cells.
Besides ethical considerations, MSCs have immunomodulatory properties, which means they can reduce inflammation and inhibit immune reactions that could lead to tissue damage or rejection. Furthermore, they typically lack MHC Class II molecules, reducing the likelihood of triggering an immune response.
Stromal cells are harvested from the patient’s tissues or healthy donor tissues: bone marrow, adipose tissue, peripheral blood, gingiva, and skin, as well as the donated placenta and umbilical cord.
Who Is a Candidate for a Stem Cell Transplant?
Stem cell therapy can be used to deal with various medical conditions, including but not limited to:
- diabetes;
- autism;
- cerebral palsy;
- ophthalmic diseases;
- age-related neurological diseases (Alzheimer’s disease, dementia);
- multiple sclerosis;
- digestive system disorders associated with tissue damage (cirrhosis, Crohn’s disease, peptic ulcer);
- autoimmune diseases (myositis, alopecia, arthritis, multiple sclerosis, myasthenia);
- conditions after a heart attack, stroke, or traumatic brain injury.
Another application of the stem cell treatment procedure is to address arthritis. Stem cell therapy treatment for knees procedure helps reduce pain and improve patient mobility.
In South Korea, there already exists a medication derived from umbilical cord blood-derived mesenchymal stem cell products, approved by the Ministry of Food and Drug Safety (MFDS) for the treatment of knee cartilage defects in patients with osteoarthritis.
The last but not least way to use stem cells is to aid in chronic obstructive pulmonary disease (COPD). It is a group of lung conditions that cause breathing difficulties, such as emphysema (damage to the air sacs in the lungs) and chronic bronchitis.
During the COPD stem cell treatment procedure, healthcare providers aim to improve pulmonary function to some extent and reduce the systemic inflammatory response. In terms of its effects, stem cell treatment today is one of the most promising therapeutic strategies for patients with COPD.
What is the process of stem cell therapy in your case?
Despite all the above, it is important to understand that stem cells are not a cure for absolutely all diseases. Sometimes a decision is made not to treat if there is too little chance that cell therapy will work. Our doctors will precisely examine the patient’s medical history and current health indicators to make an informed decision.
To learn more about the cell treatment for your particular disease, please contact our medical advisor. You can leave your question in the online chat or request a call from us.
How Does Stem Cell Procedure Work?
A straightforward way to describe the procedure for stem cell treatment is to refer to it as regenerative medicine since its purpose is to repair damaged tissues.
What is the process of stem cell therapy? As an example, in cases of head injury and stroke, healthcare providers aim to raise cells that can replace the failing neurons, which are crucial for different body functions like movement, thinking, motivation, and mood. After the preparation, specialized stem cells can be implanted into patients to contribute to repairing the injured area.
The great advantage of stem cell treatment is its usability. Stem cells can help the patient avoid permanent medical or machine support, such as ventilation. When mesenchymal stem cells (MSCs) are not differentiated into neuronal cells but are introduced into the body, they exert their effects through the secretion of various bioactive molecules. These molecules can modulate different functions in the body, depending on the specific condition being targeted.
Furthermore, timely therapy helps to improve symptoms, avoid or delay disability, and maintain a good quality of life.
Get a free online consultation
Please, contact our medical advisor to discuss your health condition with a specialist in regenerative medicine. You can also leave your contact details for a callback. It is free and confidential.

MD, Endocrinologist, Pediatrician, regenerative medicine specialist, R&D director
What Happens Before a Stem Cell Transplantation?
First, the patient turns to a specialist in our clinic to get advice about their disease. Several diagnostic studies help the doctor estimate your health status and evaluate the possibility of using cell therapy. At this stage, the decision on the most suitable type of cell product for the patient is made.
Preparing a cell product from autologous (patient’s own) material takes about 3—4 weeks, although the presence of the patient in the clinic during this period is not required.
We recommend using donor (allogeneic) cell products in situations where therapy should be started immediately or when it is impossible to use the patient’s own biomaterial for objective reasons (contraindications to anesthesia, the risk of bleeding, severe conditions).
Within 2 days before the start of cell therapy, the patient performs a general blood test and a general urine test. Next, the specialist examines the patient to make sure that there are no contraindications (such as acute infectious diseases, pathological processes in the area of administration, etc.).
To prevent possible complications, certain drugs may be prescribed in accordance with the standard treatment of the underlying disease.
The Steps of Creating a Cell Product

To gain a deeper understanding of stem cell therapy and its procedural aspects, let’s examine the process step by step.
Sampling (Harvesting)
Cell therapy starts with the sampling of the biomaterial from which the required cell type will be extracted. The approximate amount of aspirate needed to obtain cells is as follows:
- Bone marrow: 10 ml.
- Adipose tissue: 150-200 ml.
- Venous blood: 40-50 ml.
- The mucous membrane of the oral cavity (gingiva area): 3-4 mm3.
- Skin (fragment of the skin from the area behind the ear): 3-4 mm3.
Harvesting is conducted in a sterile operating room using both local and general anesthesia, particularly for obtaining stromal vascular fraction (SVF) from adipose tissue and bone marrow (BM). The biomaterial is transported to the laboratory immediately after it is harvested.
Separation and Preparation
The biopsy material is first treated and then prepared according to the standard operating procedures. Depending on the source of the aspirate, it may need to pass several steps, including filtration to separate the desired cells from other cells, and short-term incubation.
The stages of cell viability assessment include checking for viability, karyotype analysis, and the absence of infections.

Our specialists also use exosomes along with stem cells. Exosomes are extracellular vesicles secreted by stem cells that can transport proteins, RNA, and other active substances.
They possess unique characteristics that make them particularly useful for therapeutic applications, such as being enriched in bioactive molecules like growth factors and cytokines that promote tissue repair and regeneration.
The combination of stem cells and their secreted exosomes provides a more comprehensive approach and makes the treatment more effective.
Cultivation
The derived cell suspension is then cleaned and placed in special bottles with a patented culture medium, which is changed every 3–4 days. Laboratory tests for sterility are carried out.
During this period, there is an active division of stem cells and an active increase in their mass. If the cell regeneration potential is medium or high, it takes about 4 weeks to reach a sufficient number of cells.
Cryopreservation (If Required)
The cell suspension is mixed with the cryoprotectant, then transferred into cryovials and placed in a freezer controlled by precise software. To preserve the viability of the cells, the tubes are cooled according to the specified program (the cooling rate should be 1-2 degrees per minute).
At the end of the freezing program, the cryoprobes are placed in liquid nitrogen. Under these conditions, cells can be stored for an unlimited period of time. Stromal cells maintain their therapeutic potential even after cryopreservation.
If there is a need for an MMSC transplantation procedure, we never transfer cells immediately after thawing. After freezing, we work with the cells for an extended period to allow them to “wake up” and transition into the active phase of growth and multiplication. During this time, we ensure that they have tolerated the freezing process well.
Activation
Before transplantation, the cell product is prepared for clinical application. The cell product is activated by a special technology that is used by Swiss Medica to preserve the beneficial properties of the cells.
The volume of the transplant is calculated according to a formula depending on the patient’s body weight. A cell product passport indicating the source of the cells, the date of receipt, and the results of tests for microbiological purity are drawn.
Safety checks
Safety checks on stem cells and exosomes are conducted several times, including before administration, to ensure the patient’s safety and the cells’ optimal condition. We examine for:
- Absence of viruses and bacteria
- Cell characteristics
- Cell genetics
- Viability and activity.
Cell Product Introduction
The transplantation procedure takes little time compared to the time required for its creation. Depending on the underlying disease, the cell-based product is administered under medical supervision by one of the available routes:
- intravenous (through a drip);
- intramuscular: cells are injected into a muscle;
- intra-articular: cells are injected directly into a joint space;
- intrathecal: cells are injected into the cerebrospinal fluid space surrounding the spinal cord.
- and many more.

During and after the procedure, the doctor will monitor the patient’s heart rate, respiratory rate, blood pressure, and oxygen saturation level. If everything is in order, the patient soon returns to his usual life after cell transplantation.
After the Procedure of Stem Cell Treatment
What is the risk of developing side effects during the process of stem cell therapy?
It’s quite low. Multipotent mesenchymal stromal cells do not produce proteins that are recognized by the immune system, and therefore rejection is extremely rare, even in donated (allogeneic) cells. After the introduction of the cell product, a short-lived fever is the most common reaction, yet this is still relatively rare, and it passes on its own.
If you have any complaints or doubts, you can get advice from your doctor. Our specialists are always in touch, even after therapy.
How Can We Increase the Effect of Cell Therapy?
To maximize the useful properties of implanted stem cells, we use supporting methods of treatment, which speed up regenerative processes and reduce symptoms.
Depending on the disease, we apply intracellular metabolism recovery therapy, physiotherapy, spark wave, neurorehabilitation therapy, dry needling, laser or ultraviolet blood irradiation, and others.
These methods are prescribed individually, depending on the disease. In contrast to common pharmacological agents and unlike many standard treatments, they do not reduce the body’s natural ability for self-defense and do not lead to failure of metabolic and immunological processes, but they activate cell regenerative ability. It is a natural way to achieve a healthy recovery.
When Can You Expect an Improvement?
It usually takes a few weeks or months until transplanted cells start to fully take effect, although the first improvements can be felt in the days after administration. Often, reduced pain, enhanced mobility of affected joints, improved energy and activity, and improved indicators of diagnostic tests can be realized relatively quickly.
Transplanted stem cells are active for 3 months on average, 6 months at the maximum. After this period, the stem cells are no longer active, but the processes started by them continue. A complex effect is possible where not only the manifestations of the underlying disease are reduced but also the general condition of the patient is improved.
How Long Does It Take to Recover From a Stem Cell Transplant?
After undergoing stem cell therapy, there will be a recovery period. Its duration may vary depending on the type of transplantation, but with the appropriate maintenance therapy, it is at most several weeks.
Your doctor may recommend you seek a second consultation in 3 and/or 6 months after cell introduction to assess the effectiveness of the therapy. To achieve a greater and more persistent effect, the therapy can be repeated after a recommended period.
If you have any questions about the procedures and treatments using cell products, please contact our Medical Advisor. You can discuss your individual case with a specialist at the Swiss Medica Clinic.
Contact us
Get a free online consultation to learn how stem cells will work for your case, and what are the duration and cost of the therapy.

Medical Advisor, Swiss Medica doctor
List of References
Luis A Ortiz 1, Maria Dutreil, Cheryl Fattman, Amitabh C Pandey, German Torres, Kristina Go, Donald G Phinney. (2007). Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury, Proc Natl Acad Sci U S A, 104(26):11002-7. https://doi.org/10.1073/pnas.0704421104
Jaskarndip Chahal, Alejandro Gómez-Aristizábal, Konstantin Shestopaloff, Shashank Bhatt, Amélie Chaboureau, Antonietta Fazio, Jolene Chisholm, Amanda Weston, Julia Chiovitti, Armand Keating, Mohit Kapoor, Darrell J. Ogilvie-Harris, Khalid A. Syed, Rajiv Gandhi, Nizar N. Mahomed, Kenneth W. Marshall, Marshall S. Sussman, Ali M. Naraghi, Sowmya Viswanathan. (2019). Bone Marrow Mesenchymal Stromal Cell Treatment in Patients with Osteoarthritis Results in Overall Improvement in Pain and Symptoms and Reduces Synovial Inflammation, STEM CELLS Translational Medicine, 8(8), 746-757. https://doi.org/10.1002/sctm.18-0183
Yun-Tian Chen, Kang Miao, Linfu Zhou, Wei-Ning Xiong. (2021). Stem cell therapy for chronic obstructive pulmonary disease, Chinese Medical Journal 134(13):p 1535-1545, 10.1097/CM9.0000000000001596
Keshtkar S, Kaviani M, Soleimanian S, Azarpira N, Asvar Z, Pakbaz S. Stem Cell-Derived Exosome as Potential Therapeutics for Microbial Diseases. Front Microbiol. 2022 Feb 14;12:786111. doi: 10.3389/fmicb.2021.786111. PMID: 35237239; PMCID: PMC8882917.
Cristina Sanina, Joshua M Hare. (2015). Mesenchymal Stem Cells as a Biological Drug for Heart Disease: Where Are We With Cardiac Cell-Based Therapy?; Circ Res. 117(3), 229-33. 10.1161/CIRCRESAHA.117.306306
William T Tse, John D Pendleton, Wendy M Beyer, Matthew C Egalka, Eva C Guinan. (2003). Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation, 75(3), 389-97, 10.1097/01.TP.0000045055.63901.A9
M P De Miguel, S Fuentes-Julián, A Blázquez-Martínez, C Y Pascual, M A Aller, J Arias, F Arnalich-Montiel. (2012). Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med., 12(5), 574-91. 10.2174/156652412800619950
Medical Advisor, Swiss Medica doctor







